Problems of low soil pH are common throughout central and south-central Kansas. Well-drained, productive soils under good management usually become acidic over time as a natural result of high crop production. This problem typically starts in sandier soils and is exacerbated by high rates of nitrogen (N) fertilizer application over the years; making long-term continuous wheat production in central and south central Kansas especially vulnerable to this problem. However, long-term application of N also generated acid soils in other regions of the state with different soil types.
Strongly acidic soils may present several problems for wheat production. These include aluminum toxicity and in some cases manganese toxicity, as well as deficiencies of phosphorus, calcium, magnesium, and molybdenum. These problems caused by acid soils are difficult to separate one from another and are often related to root damage due to Al toxicity (Figure 1).
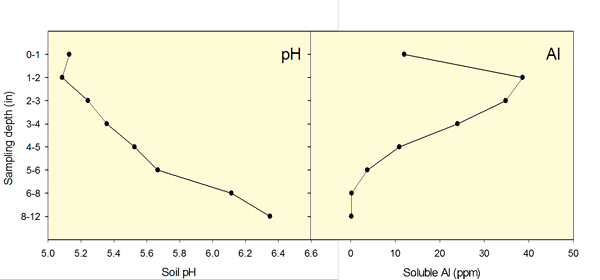
Figure 1. Soil pH stratification after long-term surface nitrogen application. Aluminum concentration in solution increases with a decrease in soil pH. Data from Dorivar Ruiz Diaz, K-State Research and Extension.
Typical symptoms of aluminum toxicity include thin stands, poor plant vigor, and purpling (Figure 2). High concentrations of aluminum will reduce root development, giving them a short stubby appearance. The roots will often have a brownish color.

Figure 2. Wheat growing on very acidic soils, such as this soil in Harper County with a pH of 4.6, is often spindly and has poor vigor. Photos by K-State Research and Extension.
In general terms, aluminum toxicity will reduce the grain yield potential of wheat when soil pH levels are below 5.5 and KCl-extractable (free aluminum) levels are greater than 25 parts per million (ppm). If aluminum levels are not high, pH levels in this range are not as much of a problem for wheat. When soil pH levels are 5.0 or less, yields start dropping off rapidly in most cases. A minimum soil pH of approximately 6.0 is needed to maximize wheat fall forage production for most wheat varieties.
Where acid soils are causing a reduction in wheat production, plant growth, and yield can be significantly improved by liming the soils and raising the pH to an optimum range.
Common questions about lime applications for wheat
- If a half-rate of lime is applied now, or in late August, will that give it enough time to benefit wheat planted in early to mid-October this year?
- Is incorporation needed to allow enough time to be effective in that situation?
Lime application may require time to react and increase soil pH. However, most of the change in pH will occur in the first 4-6 weeks after lime application. If the lime is incorporated, the effect in the upper soil profile will be relatively quick. With a lower application rate to the surface (no-till), the effect on pH would be limited to the upper 2-3 inches and would require more time to have a significant effect depending on factors such as soil texture and moisture.
- What kind of yield increases can you expect?
Several studies in Kansas have shown a significant increase in yield as well as test weight when liming acid soils (Figure 3 and Table 1). In some cases yield can easily double depending on the severity of the problem.

Figure 3. Effect of lime on wheat yields at four locations in Reno and Rice Counties. Yields averaged over two varieties – one susceptible and one tolerant to acid soils. Initial soil pH varied from 4.8 to 5.1 and lime application rates varied from 5,000 to 11,000 lbs/acre ECC. Source: Olsen, C.J. et al. Kansas Fertilizer Research 1999, SRP847.
- Should producers consider applying a lower rate of lime than what is recommended by the K-State soil testing laboratory?
It can be expensive to apply the full recommended rate of lime to soils. The yield increases from an application of the full rate of lime are likely to hold up for up to 8 years or more. But the initial cost can be quite high. Lime is a long-term investment that many producers are reluctant to make for several reasons.
If the cropping system consists of some combination of wheat, grain sorghum, corn, or sunflowers, without a legume in the rotation, then it’s not critical to use the full recommended rate of lime, particularly during years of lower grain prices. With these crops, which can tolerate somewhat lower pH levels than soybeans and alfalfa, producers may realize some benefit by applying less-than-recommended rates of lime as long as they are willing to make more frequent applications. If soybeans or alfalfa will be grown on the field in question, and if the pH level is less than 6.0, then the full rate of lime should be applied.
Table 1 below shows the effect of a lower-than-recommended rate on wheat yield and test weight. The half-rate increased yield and test weight nearly as much as the full rate in this case. However, producers should be aware that if they use lower-than-recommended rates of lime, they will need to make more frequent applications. The current crop prices require efficient and cost-effective lime application which can be achieved with the use of technology such as variable application. Soil pH can vary significantly in the field, making lime one of the inputs with the highest return to variable rate application.
Table 1. Effect of lime rate on wheat yield and test weight, Sedgwick County.
|
Lime rate |
Yield |
Test weight |
|
0 |
23 |
46 |
|
3750 (half rate) |
42 |
60 |
|
7500 (full rate) |
46 |
61 |
Variety: Karl (susceptible to acid soils). Initial soil pH: 4.7. Lime recommendation: 7500 lb ECC/acre (full rate). Source: Suderman, A.J., et al. Kansas Fertilizer Research 1994, SRP719.
- What type of lime is best to apply?
All lime materials must guarantee their ECC content and are subject to inspection by the Kansas Department of Agriculture. The purity of the lime material relative to pure calcium carbonate and the fineness of crushing are the two factors used in the determination of the Effective Calcium Carbonate (ECC) content. Lime can be from various sources and with different qualities. To ensure a standardized unit of soil-acidity neutralizing potential, we use units of ECC.
Research has clearly shown that a pound of ECC from ag lime, pelletized lime, water treatment plant sludge, fluid lime, or other sources is equal in neutralizing soil acidity. All lime sources have very limited solubility and must be incorporated and given time to react with the acidity in the soil to effect neutralization.
Therefore, when selecting a lime source the cost per pound of ECC should be a primary factor in source selection. Such factors as rate of reaction, uniformity of spreading, and availability should be considered, but the final pH change will hinge on the amount of ECC applied.
Other recommendations to increase yields in acid soils include the use of aluminum-tolerant wheat varieties and applying phosphate fertilizer with the seed to tie up aluminum and reduce toxicity. These management practices can certainly help to maintain yields and may be the best alternatives for some producers. However, there is only one long-term solution to low soil pH levels: liming.
Dorivar Ruiz Diaz, Nutrient Management Specialist
ruizdiaz@ksu.edu
Tags: wheat soil pH liming aluminum toxicity