With the current high prices of commercial fertilizers, it is even more important to implement management practices that minimize nutrient loss and maximize crop uptake. Some common nitrogen (N) containing fertilizers used in Kansas are composed of urea. This article explains some of the science behind what happens to urea when it is applied to soil. The information is taken from a new KSRE publication, MF894 Management Practices Affecting Nitrogen Loss from Urea. The full article can be viewed at: https://bookstore.ksre.ksu.edu/pubs/MF894.pdf.
Urea fertilizers range in composition from pure, dry, granular urea (46-0-0) to products that are mixtures of urea and other sources of N and/ or phosphate and potash. The most common mixture of urea with other N fertilizers is the liquid urea-ammonium nitrate solution (UAN), which in Kansas is most often sold as a solution containing 28% N. It also may be sold as a 32% N solution. Approximately half of the N in UAN is urea.
While cost advantages favor increased use of urea, questions are often raised about its availability to crops compared to other N sources and its potential for loss when applied to the soil surface and not incorporated by tillage or irrigation. Chemical reactions of urea and ammoniacal N (ammonia and ammonium) in soil, and soil, climate, and management factors that affect the performance of urea need to be understood for proper use.
Reactions of Urea in Soil
Urea applied to the soil reacts with water and the soil enzyme urease and is rapidly converted to ammonium. This conversion, shown with the chemical reaction below, is called urea hydrolysis. In this reaction, hydrogen ions (H+) are consumed, causing the soil pH near the fertilizer to rise. If the pH rises above 7, a significant amount of gaseous ammonia can form in soil for a few days following urea application. When urea is surface‑applied, the formation of ammonia at the soil surface from urea hydrolysis may allow some ammonia to be lost, and if urea is banded with the seed, some plant damage may occur because of ammonia toxicity. The severity of both processes depends largely on the concentration of ammonia formed in the soil.
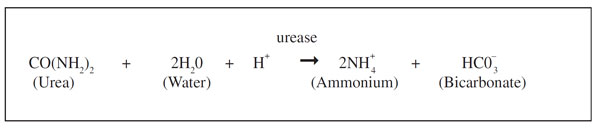
The concentration of ammonia in the soil from urea hydrolysis depends on a number of factors. The most important are:
1) The rate of urea applied. Larger urea applications generally result in more hydrolysis and higher ammonia concentrations in soil. Band applications also concentrate the urea in smaller volumes of soil, which can result in more ammonia formation at the site of fertilizer placement; however, this does not mean that ammonia loss will be greater from surface‑banded urea, since the hydrolysis rate may be reduced (see number 3).
2) The pH at the soil surface for the first three to five days following urea application. The higher the pH during this time, the more ammonia will be formed. Soils vary in their ability to resist the increase in pH due to the amount of hydrogen ions they contain. Soils with relatively large amounts of clay and organic matter, and low pH before urea is applied have relatively large amounts of hydrogen ions. Less ammonia will be formed on these soils. At the other extreme, soils that are sandy and low in organic matter, especially those with a high pH, allow more ammonia to be formed from urea hydrolysis.
3) The speed (rate) of urea hydrolysis in soils. Fast urea hydrolysis reduces the time available for urea and ammonium (and any gaseous ammonia) to diffuse deeper into the soil when surface-applied (or away from the seed in case of seed-placed urea). When the time for diffusion into the soil is reduced, the ammonium will be more concentrated at the surface, the pH will be higher, and more ammonia will form. The factors affecting the rate of hydrolysis that are most likely to change from field to field include the amount of urease enzyme in the soil, soil temperature, and soil moisture. Since band application reduces the contact between fertilizer and soil urease, this method slows the rate of urea hydrolysis.
Weather Conditions at and Shortly after Application
Two weather-related factors, temperature and moisture, greatly affect urea hydrolysis rates and ammonia loss from surface-applied urea fertilizers. If a choice is possible, apply urea fertilizers when temperatures are cool. Wheat and cool-season grasses can be fertilized in late winter to good advantage, rather than late spring when temperatures begin to rise. Even though losses are usually not large with later application, the early application is preferred. Although application under cool or cold conditions is preferred, there is potential for loss of fertilizer in storm runoff should an unusual winter rainstorm or quick snowmelt occur when soils are frozen. Poor fertilizer performance has been observed in a few instances when these somewhat rare weather events occurred. It is best to avoid application of fertilizer to frozen soils, if there is a high probability of rapid warming conditions with rainstorms and runoff. If the surface soil is partially thawed at fertilizer application time or if it thaws soon after application, the fertilizer will dissolve and diffuse into the soil within a day or two. If storms and runoff then follow, losses will be small.
Application is also better under dry surface soil conditions than under wet conditions to avoid ammonia loss. Usually, the surface of a well-drained soil dries quickly in Kansas weather. Soils with high water tables, however, may stay moist near the surface for longer periods of time. Lower parts of a field that stay wet for long periods of time may also experience some problems with ammonia loss, whereas well-drained areas of a field may not. Somewhat higher rates of application on these wetter areas could increase production by offsetting some N loss.
Reference: Perin, V., Santos, E.A., Lollato, R., Ruiz Diaz, D.A., Kluitenberg, G.J. 2020. Impacts of ammonia volatilization from broadcast urea on winter wheat production. Agronomy Journal. 2020; 112: 3758– 3772. https://doi.org/10.1002/agj2.20371
Dorivar Ruiz Diaz, Soil Fertility Specialist
ruizdiaz@ksu.edu
Tags: nitrogen fertilizer urea