Understanding spray water quality is a crucial first step for successful herbicide applications. Much of the water used for herbicide applications in Kansas is inherently “hard,” and the quality of water from some wells is decreasing as water resources decrease. This article will focus on the effects of water quality on herbicide efficacy, particularly in postemergence applications. Three main factors affect water quality in Kansas - turbidity, pH, and hardness, and we’ll look at each more closely.
Turbidity is defined as the clarity of water and is mostly affected by larger particles suspended in the water, such as algae, clay, minerals, or organic matter. Poor turbidity is more likely to exist where surface waters such as ponds, streams, or rivers are the source of spray water. These suspended particles interact with many of our herbicides and can influence uptake by the plants, chemical changes at the site-of-action (SOA), and decrease the performance of sprayer equipment. Its effect is severe on cationic (positively charged) herbicides (paraquat and diquat) and herbicides with low soil mobility (glyphosate). If turbidity is an issue, chelating agents may be available for use in holding ponds or tanks prior to mixing in the spray tank.
Spray water pH is a measure of acidity or the presence of hydrogen ions. Pure water is neutral, having a pH of 7.0, whereas values below 7 are considered acidic, and values above 7 are basic. Kansas surface and subsurface water pH typically ranges from 6.5 to 8.5. Generally, pH values of spray water in Kansas do not have a large effect on the herbicides commonly applied. The efficacy of weak-acid herbicides (glyphosate, glufosinate, clethodim, sethoxydim, bentazon, and 2,4-D) is generally improved with acidic water pH. In contrast, the efficacy of sulfonylurea herbicides (group 2) is negatively impacted by acidic pH. These sulfonylurea herbicides and HPPD herbicides (Table 1) tend to be more effective (more soluble) at a pH above 7, whereas flumioxazin degrades rapidly in spray water with a pH greater than 7.8. Additionally, very low pH values can cause salts of certain herbicides (glyphosate, 2,4-D, others) to precipitate out. Also, it is important to keep in mind that dicamba volatility increases in acidic solutions with a pH lower than 5.0. Unless a known water source is extremely acidic or basic, pH-modifying agents are not usually necessary.
Table 1. Common herbicides used in Kansas.
|
Herbicide group |
Site-of-action |
Examples |
Brand names |
|
Group 1 |
ACCase inhibitors |
Quizalofop, sethoxydim |
FirstAct, Select, others |
|
Group 2 |
ALS inhibitors |
Metsulfuron, imazamox |
Ally, Beyond, Imiflex |
|
Group 4 |
Synthetic auxins |
Dicamba, 2,4-D, fluroxypyr |
Banvel, Starane, others |
|
Group 9 |
EPSP inhibitor |
Glyphosate |
Roundup, others |
|
Group 10 |
Glutimine inhibitor |
Glufosinate |
Liberty, others |
|
Group 14 |
PPO inhibitors |
Flumioxazin, saflufenacil, sulfentrazone |
Valor, Sharpen, Authority, others |
|
Group 27 |
HPPD inhibitors |
Mesotrione, tembotrione |
Callisto, Laudis, others |
Water hardness is one of the largest issues impacting herbicide applications in Kansas. Hardness is caused by the presence of positively charged minerals (cations) suspended in the water, particularly calcium (Ca), sodium (Na), magnesium (Mg), potassium (K), and iron (Fe). Because many of our herbicides, notably glyphosate and glufosinate, form weak acids (negatively charged) when mixed in water, cations can bind to herbicide molecules, thus changing how the herbicide is absorbed by the plants and altering its ability to bind at the site of action.
Water hardness can be classified into the following groups based on the total number of cations present expressed in parts per million (ppm):
- Soft = less than 75 ppm
- Moderately hard = 75 to 150 ppm
- Hard = 150 to 300 ppm
- Very hard = greater than 300 ppm
Figure 1 shows the cation concentrations from four wells in Finney County, where all but one (Finnup) had total cation concentrations above the 300 ppm level. Furthermore, cation concentrations varied greatly even for these wells, which were within three miles of each other. To combat hard water antagonism, water conditioners such as ammonium sulfate (AMS) are recommended with many of our postemergence herbicides. When added to the water prior to the herbicide, AMS produces ammonium ions which bind to the herbicide and prevent other cations from interacting with it. Recommended rates of AMS range from 8.5 to 17 pounds per 100 gallons of spray water. North Dakota State University researchers developed a simple formula for calculating AMS needs based on the presence of cations in the water and their ability to interact with herbicides:
(0.002 x ppm K)+(0.005 x ppm Na)+(0.009 x ppm Ca)+(0.014 x ppm Mg)+(0.042 x ppm Fe) = lbs AMS/100 gallons
Plugging in the cation numbers from the domestic well in our graph, we get the following equation:
(0.002 X 9)+(0.005 X 190)+(0.009 X 230)+(0.014 X 120)+(0.042 X 0) = 4.7 lb AMS/100 gallons.
So, approximately 5 pounds of AMS per 100 gallons of water is needed to counteract the cations in this example. A handy Excel-based version of this formula can be found at the website: https://sprayers101.com/wp-content/uploads/2022/02/AMS-Calculator-Sprayers101-1.xlsx
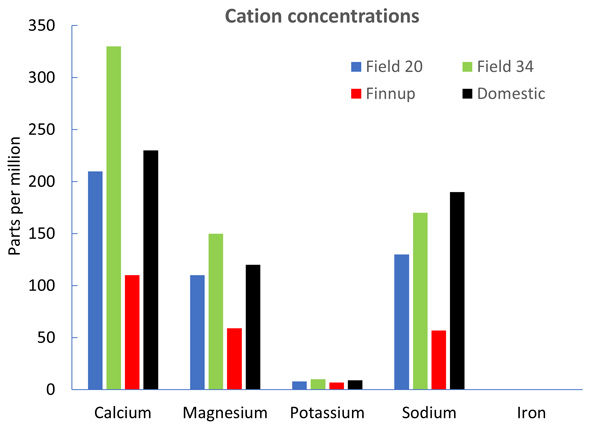
Figure 1. Cation concentrations in water samples from four wells at the Kansas State University Research & Extension Center in Garden City, KS.
There are also liquid AMS replacement products on the market. These products are very convenient and easy to use compared to dry AMS. However, be aware that these products vary greatly in their composition, and it is important to ensure that sufficient ammonium ions are being added to properly condition the hard water. Previous research at K-State indicated that many of the products can be equal to dry AMS, but most are more expensive, and some did not perform as well as dry AMS. Another important point to remember is that in addition to being a water conditioner, AMS is also an adjuvant that influences herbicide uptake by plant leaves. Interestingly, the weeds we are targeting can also affect our spray operations. Species such as velvetleaf, lambsquarters, and sunflower tend to have greater concentrations of cations on their leaf surfaces than other species, and this can lead to greater herbicide antagonism.
There are other ways to combat water quality issues for spraying. If practical, using treated water from a municipal source can limit the negative influences of turbidity, pH, and hardness. However, there may be additional costs associated with this, and travel distances may make it unfeasible. Some producers have opted to install commercial reverse osmosis (RO) systems to combat poor water quality. This can be an effective method, but it comes with costs as well. If thinking about investing in an RO system, consider the initial purchasing cost, cost of filter maintenance, cost of storage for the treated water, and disposal of the effluent water produced. In cases where water hardness is the primary issue, this can also be overcome by increasing the rates of the herbicide in question. As herbicide concentration increases, a smaller percentage of the active ingredient is tied up by the cations in a given volume of water. Conversely, using a lower spray gallonage may be an option as well. Less water in the tank means fewer cations to interact with the herbicide. Always consult the herbicide label to ensure maximum herbicide rates are not exceeded and the minimum gallon-per-acre volumes are met.
Summary
Water quality has a large impact on herbicide efficacy. As water resources decline, water quality can and does change over time. A proper water test is the best way to understand what characteristics are influencing our spray applications and how to mitigate those effects. K-State, as well as several commercial providers, can conduct water tests for a nominal charge.
During the summer of 2025, researchers at K-State will be collecting spray water samples from around the state to test for quality. If you are interested in participating in this study, please contact one of the authors below, your local Extension agent, or your regional Extension Specialist.
For an in-depth discussion on spray water quality, check out the War Against Weeds podcast on the subject. It can be found at: https://waragainstweeds.libsyn.com/s9-e2-spray-solution-quality
More information can also be found at: https://bookstore.ksre.ksu.edu/pubs/2025-chemical-weed-control-for-field-crops-pastures-rangeland-and-noncropland_CHEMWEEDGUIDE.pdf
References:
War Against Weeds podcast Season 9, episode 2, https://waragainstweeds.libsyn.com/s9-e2-spray-solution-quality
2025 North Dakota Weed Control Guide, J. Ikley, et al. publication W253-25.
The use of trade names is for clarity to readers and does not imply endorsement of a particular product, nor does exclusion imply non-approval. Always consult the herbicide label for the most current use requirements.
Patrick Geier, Weed Scientist, Garden City
pgeier@ksu.edu
Sarah Lancaster, Extension Weed Management Specialist, Manhattan
slancaster@ksu.edu
Jeremie Kouame, Weed Scientist, Hays
jkouame@ksu.edu
Logan Simon, Southwest Area Agronomist, Garden City
lsimon@ksu.edu