Soybean cyst nematode (SCN) is the number one yield-limiting pathogen of soybean and is distributed in fields throughout eastern and central Kansas. This pest has been identified in 64 counties that account for more than 85% of the state’s soybean production (Figure 1). A recent 2-year survey of soybean growers in Kansas found that fewer than 10% of growers test for SCN.
Visible symptoms of SCN can easily be confused with other issues in soybean production. SCN also weakens plants, making them more susceptible to other diseases such as Sudden Death Syndrome (SDS). Because SCN populations can increase rapidly and persist in the soil for years, regular monitoring is essential. Sampling helps determine whether current management strategies, such as crop rotation and use of resistant soybean varieties, are effectively reducing nematode numbers. Without testing, populations can build silently, resulting in significant long-term yield losses.
The best time to test for SCN is immediately after harvest, when soil conditions are favorable for sampling and before making seed and rotation decisions for next season. Confirming SCN presence, estimating population levels, and monitoring the effectiveness of resistant varieties form the foundation of a successful integrated management plan.
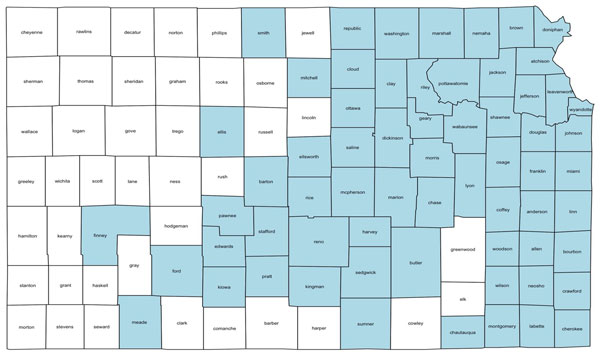
Figure 1. As of October 1, 2025, SCN was identified in 62 Kansas counties that produce >85% of Kansas soybeans. Map by William Rutter and Chandler Day, K-State Research and Extension.
To collect a SCN sample, you will need:
- A soil probe (or sharpshooter spade)
- A bucket
- A labeled bag. The label should include the following information:
- Field identification (i.e., Field ID: North Farm, near Doe Creek)
- Size of the area being sampled (i.e., 20 acres)
- Crop rotation history (i.e., soybean, corn, and soybean)
Recommended field pattern for sample collection:
If your field is fairly uniform, divide it into quadrants or sections for your SCN sample collection. Fields with different cropping histories or soil types should be sampled separately. For each quadrant, collect 10 to 20 cores to a depth of 6 to 8 inches.
Walk the area in a systematic pattern, such as a “Z” pattern (Figure 2). Collect a total of 10 to 20 soil cores, emptying each into the bucket after collection. All core samples should be mixed well to account for variation between cores. After mixing, collect 1 pint of soil (~2 cups) in a labeled plastic bag and seal.
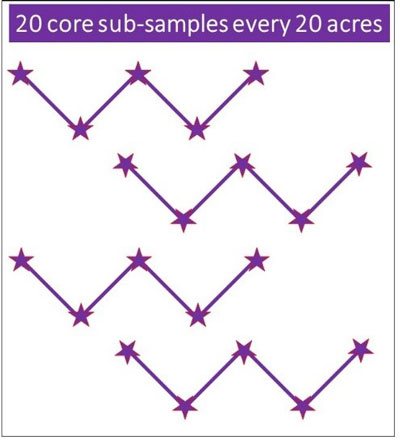
Figure 2. Example of a good sampling pattern for collecting soil to test for SCN.
Preparing and sending samples
When sending your samples to the diagnostic lab, make sure to:
- Ship overnight or as fast as possible
- Avoid leaving bags in the sun
- Send the samples to the Plant Disease Diagnostic Lab in the K-State Plant Pathology Department.
- Fill out the Plant Disease Diagnostic Check sheet at https://www.plantpath.k-state.edu/extension/diagnostic-lab/documents/2021_PP_DiseaseLabChecksheet.pdf.pdf
Shipping address:
K-State Plant Disease Diagnostic Lab
4032 Throckmorton PSC
1712 Claflin Road
Manhattan, KS 66506
clinic@ksu.edu
785-532-1383
For a step-by-step demonstration, watch this short video: https://youtu.be/b6Eo0isI1I0
Remember, your results will only be as good as the sample you send to the lab!
Diagnostic testing fees:
- Internal clients (KSRE agents): $25
- External clients (crop consultants, farmers, and others): $35
More details about fees are available at: https://www.plantpath.k-state.edu/extension/plant-disease-diagnostic-lab/services-and-fees.html
What to do if you find SCN in your fields
There are management options that can help reduce SCN populations and reduce the yield losses in your fields. You can enter the SCN counts provided by the K-State Disease Diagnostic Lab, along with your soil parameters, into the online SCN Coalition Profit Checker tool: https://www.thescncoalition.com/profitchecker/calculator/. The output will help you decide what the most economical SCN management strategy is for your fields next season.
Soil sampling for fertility, too?
If you plan to sample for soil fertility, you can save time by collecting both sets of samples during the same field visit. The sampling procedure is nearly identical, simply split the soil into two portions:
- One for the Soil Testing Laboratory
- One for the Plant Disease Diagnostic Laboratory
Keep the soil for SCN testing field-moist, and follow the handling and shipping instructions above. More information on soil fertility testing is available at: https://www.agronomy.k-state.edu/outreach-and-services/soil-testing-lab/
Rodrigo Onofre, Row Crop Plant Pathologist
onofre@ksu.edu
Chandler Day, Row Crop Diagnostician
chandlerday@ksu.edu
William Rutter, Plant Nematologist
wrutter@ksu.edu