Soils across most of Kansas are still running above 50 degrees F at the 4-inch depth in most locations (Figure 1). It is best to delay application of anhydrous ammonia until soil temperatures drop below this threshold. Applying anhydrous ammonia in the fall ahead of the next corn crop has some appeal to producers. For one thing, fall fertilizer application spreads out the workload so there’s more time to focus on corn planting in the spring. Secondly, wet conditions in the spring sometimes prevents producers from applying lower-cost anhydrous ammonia ahead of corn planting, and forces them to apply more expensive sources after planting. Equally important for many producers have been issues with anhydrous ammonia availability at times in the spring.
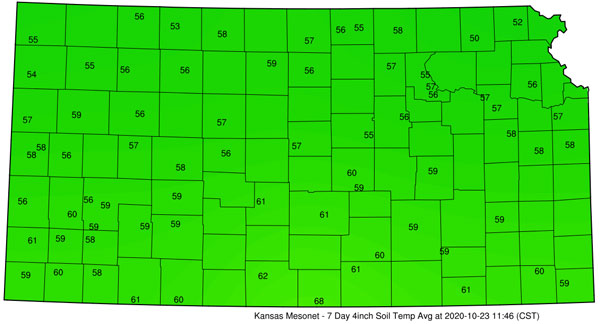
Figure 1. Average soil temperature at 4 inches for the 7-day period ending on October 23, 2020. Soil temperatures in individual fields in any given area will vary with differences in vegetative cover, soil texture, soil moisture, and other factors. (Kansas Mesonet)
Despite those advantages, producers should be aware that there is potential for higher nitrogen (N) loss in the spring following a fall application, as a result of nitrification of the ammonium during late winter and very early spring and subsequent leaching, or denitrification.
Reactions of anhydrous ammonia in the soil
When anhydrous ammonia is applied to the soil, a large portion of the ammonia is converted to ammonium (NH4+), and can be bound to clay and organic matter particles within the soil. As long as the nitrogen remains in the ammonium form, it can be retained on the clay and organic matter, and does not readily move in most soils except sandy soils with very low CEC, so leaching is not an issue.
At soil temperatures above freezing, nitrification occurs - ammonium is converted by specific soil microbes into nitrate-N (NO3-). Since this is a microbial reaction, it is very strongly influenced by soil temperatures. The higher the temperature, the quicker the conversion will occur. Depending on soil temperature, pH, and moisture content, it can take 2-3 months or longer to convert all the ammonia applied in the fall to nitrate.
By delaying application until cold weather, most of the applied N can enter the winter as ammonium, and over-winter losses of the applied N will be minimal.
Producers should wait until soil temperatures are less than 50 degrees F at a depth of 4 inches before applying ammonia in the fall or early winter. Nitrification does not cease below 50 degrees F, but rather soils will likely become cold enough to limit the nitrification process. In many areas of Kansas, soils may stay warmer than 50 degrees well into late-fall and only freeze for short periods during the winter.
The use of a nitrification inhibitor can help reduce N losses from fall N applications under specific conditions, particularly during periods when soil temperatures warm back up for a period after application.
One should also consider soil physical properties when considering fall application. Fall applications of N for corn should not be made on sandy soils prone to leaching, particularly those over shallow, unprotected aquifers. Rather, fall N applications should focus on deep, medium- to heavy-textured soils where water movement through the profile is slower.
When is N lost?
When considering fall application of N, keep in mind that loss of N during the fall and winter is not normally a problem in Kansas. The conversion of “protected” ammonium to “loss prone” nitrate during the fall and winter can be minimized by waiting to make applications until soils have cooled, and by using products such as nitrification inhibitors. The fact that essentially all the N may remain in the soil as ammonium all winter, coupled with our dry winters, means minimal N is likely to be lost over winter.
However, soils often warm up early in the spring and allow nitrification to get started well before corn planting. Generally, if the wheat is greening up, nitrification has begun! Thus, one of the potential downsides of fall application is that nitrification can begin in early March, and essentially be complete by late May and June.
Summary
If anhydrous ammonia is to be applied in the fall, there are a number of factors that must be considered, including soil texture, temperature, and soil moisture. Consider the following guidelines:
- Do not apply anhydrous ammonia in the fall on sandy soils (see companion article in this eUpdate for more information).
- On silt loam or heavier-textured soils, wait to apply anhydrous ammonia until soil temperatures at the 4-inch depth are below 50 degrees F (records indicate in most years this will be in November).
- Use a nitrification inhibitor with anhydrous ammonia to help reduce fall nitrification rates.
- To check the soil temperature in your area visit the K-State Research and Extension Weather Data Library at: http://mesonet.k-state.edu/agriculture/soiltemp/
Dorivar Ruiz Diaz, Nutrient Management Specialist
ruizdiaz@ksu.edu
Mary Knapp, Weather Data Library
mknapp@ksu.edu